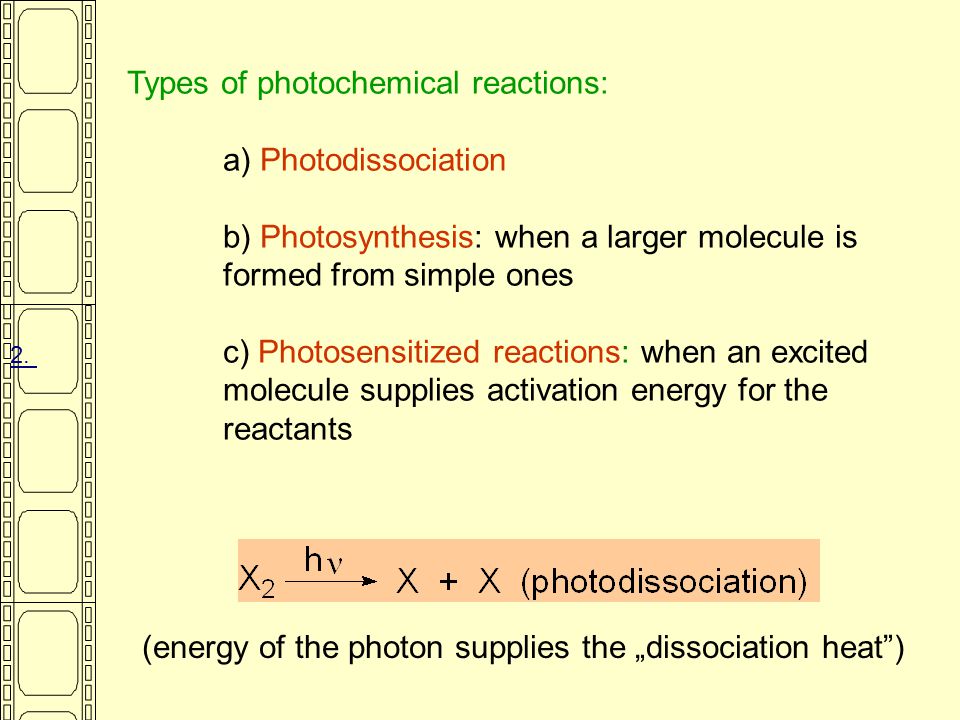
Explain Different Types of Photochemical Reactions
These reactions are sometimes described as examples of photocatalysis - reactions catalysed by light. Migration of a σ-bond.

Photochemical Reaction Definition Theoretical Concepts
They are electrocyclic ring opening in the vitamin D synthesis and thermoreversible hydrogen shift in the ultraviolet stabilization of plastics.

. These reactions involve absorption or evolution of heat energy. May be intermolecular or intramolecular. To learn more about types of organic reactions read the below article.
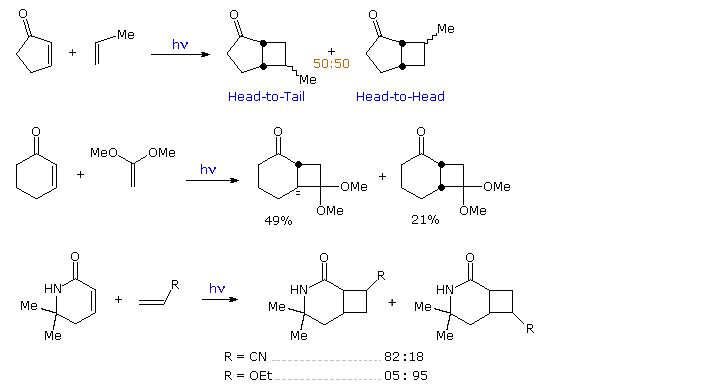
A cyclic product is formed. Commonly these processes are classified depending on the type of chemistry induced by photon absorption eg photoisomerization hydrogen atom abstraction photosensitized reactions etc. Each distinct energy corresponds to an electronic state of the molecule.
Addition reactions elimination reactions substitution reactions pericyclic events rearrangement reactions photochemical reactions and redox reactions are the most common types of organic chemistry reactions. The reactions in which the reactant forms an intermediate and the intermediate forms the product in one or many subsequent reactions are called as consecutive or sequential reactions. These reactions can take place in light as well as in dark.
By using the Beer-Lambert law we may write for the absorbance measured at the irradiation and observation wavelength at time t. The type of rotation determines whether the cis or trans isomer of the product will. Different Types of Chemical Reactions.
A photochemical reaction is a form of chemical reaction in which the reactants get energy as photons while a thermal reaction is a form of chemical reaction in which the reactants get energy as heat. O 3 hν O 2 O. The energy is said to be quantized.
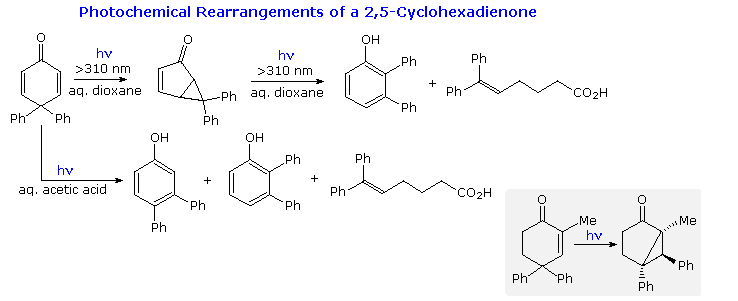
In such reactions the product is not formed directly from the reactant. Since it is the electrons that provide the bonding forces that hold atoms together into molecules if the distribution of electrons within a molecule changes drastically the. A 12-rearrangement is another subsequent type of organic reaction.
It is better to use the term photochemical and keep the word catalysis for reactions speeded up by actual substances rather than light. 2 Electrocyclic reactions 1-1. This is the key difference between photochemical and thermal reaction.
However for convenience Photochemical reactions are classified as Cis Trans Isomerization Sigmatropic Rearrangements Electrocyclic Rearrangements and Structural Rearrangements. A Photodissociation b Photosynthesis. 16 rows Hydrogen abstraction and addition to double bonds are typical reactions.
A reaction in which two or more reactants combine to form a single product is known as a combination reaction. Structural Rearrangements result from intramolecular Cycloadditions. 4 Group transfer reactions 1-1.
Up to now there are only two major kinds of photochemical isomerization reactions. Those reactions in which two compounds by the exchange of ions to form two new compound are called double displacement reactions. Difference between Photochemical and thermal Reaction- Photochemical Reaction Thermal Reaction These reactions involve absorption of light radiations.
There are different types of electrocyclic reactions because it is a broad branch of organic chemistry. Intermolecular transfer of a group. Chemical reactions involve breaking old chemical bonds between the atoms of reacting substances and then making new chemical bonds.
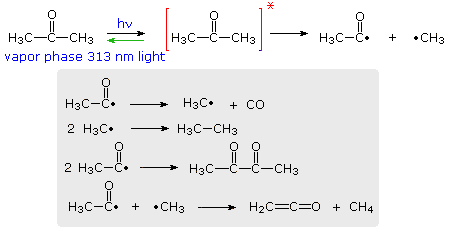
Theories of reaction rates do not agree well on complex reactions. Besides photolysis several other types of photochemical mechanisms are known such as those briefly outlined in Table 141. Absorption of energy causes the breaking of the bonds present in the reacting substance which decomposes to give the product.
Reactions can be either ring-opening or ring-closing electrocyclization. In the following we will however focus only on some of the. Reactions can be either photochemical or thermal.
When a larger molecule is formed from simple ones c Photosensitized reactions when an excited molecule supplies activation energy for the reactants. An example of a photo decomposition reaction is the decomposition into dioxygen and an oxygen radical as represented by the chemical equation provided below. One type of photochemical reaction is the dissociation of a molecule into two fragments.
Chemical reactions involving organic molecules are known as organic reactions. This is a good example of a photochemical reaction - a reaction brought about by light. Some categories include photochemical reactions thermal reactions ring-opening or ring-closing reactions etc.
Depending on the type of reaction photochemical or thermal and the number of pi electrons the reaction can happen through either a conrotatory or disrotatory mechanism. The course of a photochemical reaction is often monitored spectrophotometrically at wavelengths different from the irradiation wavelength. A photodecomposition reaction is a type of decomposition reaction in which the reactant is broken down to its constituents by absorbing energy from photons.
A double displacement reaction usually occurs in solution and one of the products being. Students understand the characteristics of a decomposition reaction different types of such reactions. The 5 primary types of chemical reactions are.
Types of Complex reaction. Presence of light is the primary requirement. The processes in which a substance or substances change to produce new substances with new properties are known as chemical reactionsThere are different types of chemical reactions happening around us.
Cycloaddition reactions 2 -2. The chemical nature of a molecule is primarily described by the behaviour of its electronsAn important facet of quantum mechanics is that the total energy of a molecules electrons its electronic energy can take on only certain distinct values. Migration of a σ-bond from one molecule to another.
Types of photochemical reactions. CuSO 4 aqZnsZnSO 4 aqCus In this reaction zinc displaces copper from copper sulphate solution. A classic example of an electrocyclic reaction is the thermal ring-opening reaction of the cis-isomer of 34-dimethylcyclobutene.
Most decomposition reactions require energy either in the form of heat light or electricity.
Photochemical Reactions Of Photosynthesis Are Connected By An Electron Download Scientific Diagram

Photochemical Reaction Definition Theoretical Concepts

Examples Of Continuous Flow Photochemical Reactions Driven By Visible Download Scientific Diagram

Schema Of A Photochemical Reaction During Photodynamic Therapy Download Scientific Diagram

Photochemical Reaction Definition Examples Applications

Example Of Photochemical Reaction And Electrochemical Reaction Brainly In
Organic Photochemistry Authorstream

Photochemical Reaction Definition Examples Applications

Is Light A Reactant In Photochemical Reactions Quora

Photochemical Reactions Of Photosynthesis Are Connected By An Electron Download Scientific Diagram
Chemical Kinetics Photochemical Reactions The Chemistry Guru

Rate Of Chemical Reaction And Chemical Equilibrium Ppt Video Online Download
Photochemistry Ppt Video Online Download

Question Video Definition Of Photochemical Reactions Nagwa

Comments
Post a Comment